Cow’s milk protein allergy resolved with the use of Alfamino® Infant formula after other nutrition regimens failed
Catherine Brigman, MD1, Aimee Henrikson, MPH, RD, LDN2
1Pediatric Specialists of Texas, San Antonio, TX; 2Medical Scientific Liaison, Nestlé Health Science
Abstract:
Three month old female presented with reflux and skin rash after hospitalization for apnea during feeding and was diagnosed with cow’s milk protein allergy (CMPA), reflux and eczema. This patient also had a complicated history including difficulty latching during breastfeeding, tongue tie (treated and resolved), swallowing delay, failure to thrive, reflux and eczema. Feeding history, since birth, included combination of breastmilk + Gentlease® for the first 11 weeks of life, a switch to breastmilk + Nutramigen® (with the addition of Prevacid®) for 8 days up until hospital admission for apnea. During hospitalization, this patient was switched to Neocate®. After experiencing worsening reflux and continuing eczema on Neocate®, the patient was switched to Elecare®. Dermatologist prescribed hydrocortisone, with no improvement of rash. A second episode of apnea occurred while on Elecare® and the patient was switched to Alfamino® Infant. With switch to Alfamino® Infant, rash resolved, reflux improved, no additional episodes of apnea were reported and growth improved.
Case Report
Detailed Description of Patient:
Patient was referred to the pediatric gastroenterologist at 3 months of age after hospitalization for apnea. She was born full term and had a complicated history, since birth, including difficulty latching during breastfeeding, tongue-tie, swallowing delay, failure to thrive, reflux and eczema. At time of referral, rash was noted on the patients face, trunk, arms and legs (over 90% of her body) and reflux was reported multiple times per day.
Patient’s feeding history started with consuming a combination of breastmilk + Enfamil Gentlease® (partially hydrolyzed formula; Mead Johnson Nutrition) for the first 11 weeks of life and transitioned to breastmilk + Nutramigen® (hypoallergenic extensively hydrolyzed formula (EHF); Mead Johnson Nutrition) with daily intake of Prevacid® (one time per day). Patient was hospitalized approximately one week later after an episode of apnea during feeding. During hospitalization, patient was switched to Neocate® (hypoallergenic amino acid-based formula (AAF); Nutricia North America) due to suspected cow’s milk protein allergy that did not resolve with Nutramigen®. Post discharge, patient continued to have reflux and eczema with Neocate®.
At 3 months of age when referred to the pediatric gastroenterologist, patient weighed 4.4 kg (0.8% ile) and measured 56 cm in length (2% ile). Pediatric gastroenterologist diagnosed patient with cow’s milk protein allergy, reflux and eczema and recommended a switch from Neocate® to Elecare® (hypoallergenic, AAF; Abbott Laboratories) due to continued symptoms. Patient was also referred to dermatology and started using a topical hydrocortisone cream; however, her rash did not improve. Patient then experienced another apnea episode while on Elecare® and the pediatric gastroenterologist recommended a formula switch from Elecare® to Alfamino® Infant formula (hypoallergenic, AAF; Nestle HealthCare Nutrition). After the patient switched to Alfamino® Infant, her rash resolved, she had no additional episodes of apnea and her growth improved.
At 4 months of age, patient started on rice cereal, supplemented by 2 oz of baby food (squash and sweet potato) 3 times daily. She continued to consume complementary foods and Alfamino® Infant formula, and saw continued growth with no additional symptoms of allergy. At 13 months of age, Alfamino® Infant was discontinued and almond milk was introduced. Since patient stopped consuming Alfamino® Infant, her growth has slowed, she has been having diarrhea 3-4 times per day and subsequent diaper rash. These issues are currently being evaluated by her medical team.
Growth History:
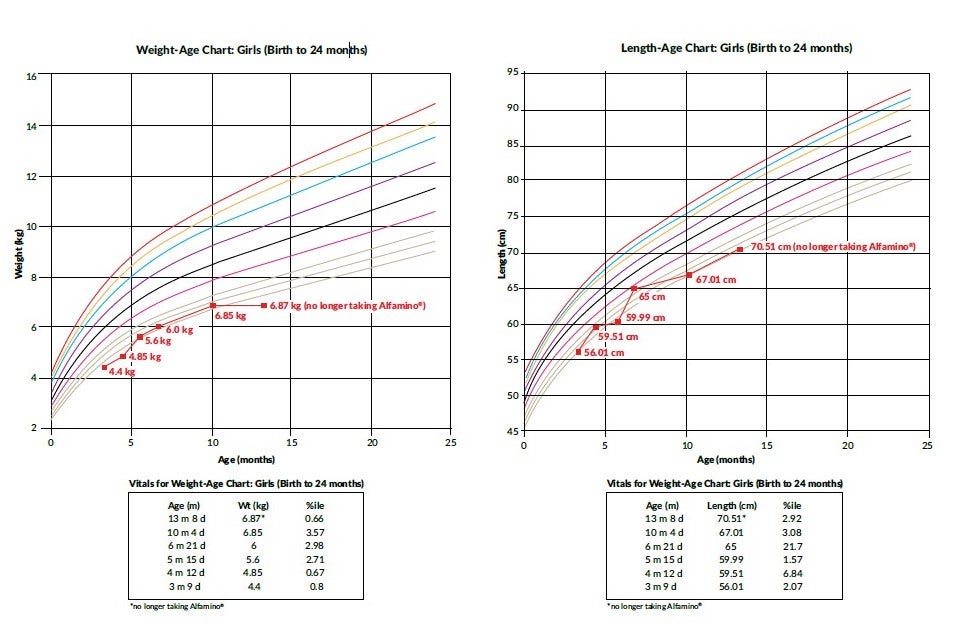
Birth weight: 3.0 kg; birth length: 49.1 cm
3 months (Pediatric gastroenterologist appointment post-hospitalization): 4.4 kg (.8% ile), 56 cm (2% ile)
4 months: 4.85 kg (.67% ile), 59.5 cm (6.8 % ile)
7 months: 6 kg (2.9% ile), 65 cm (21 % ile)
10 months: 6.85 kg (3.5% ile), 67 cm (3% ile)
13 months (no longer taking Alfamino®): 6.87 kg (.66% ile), 70.5 cm (2.9% ile)
Growth Charts:
Nutrition Intervention:
- Several nutrition interventions were attempted to alleviate allergy symptoms
- Prior to introduction of Alfamino® Infant, the following nutrition interventions were attempted:
- Patient started with combination of breastmilk and partially hydrolyzed formula (Gentlease®) for the first 11 weeks
- Patient then transitioned to a combination of breastmilk and hypoallergenic extensively hydrolyzed formula (EHF) (Nutramigen®)
- Patient was then transitioned to hypoallergenic, amino acid formula (AAF) (Neocate®)
- Patient was then transitioned to a different hypoallergenic AAF (Elecare®)
- Finally, due to persistence of symptoms on the previous nutrition interventions, patient was transitioned to hypoallergenic AAF (Alfamino® Infant)
- Alfamino® Infant was the only hypoallergenic formula that resulted in alleviation of symptoms of CMPA
Findings/ Outcome:
- With the use of Alfamino® Infant formula, eczema resolved, no additional episodes of apnea occurred, reflux improved and growth improved until 13 months of age (at which point Alfamino® Infant had been discontinued for several weeks)
Discussion:
- The recommended treatment of CMPA in a formula fed infant is to switch from an intact protein formula to a hypoallergenic, extensively hydrolyzed formula (EHF). If symptoms persist, the recommendation is to then switch to a hypoallergenic, amino-acid based formula (AAF)1,2. In this case, three HA formulas (one EHF and two AAF) were attempted with no resolution of symptoms. When Alfamino® Infant was consumed, the symptoms resolved.
Conclusion:
- A 3 month old infant diagnosed with CMPA had persistent unresolved allergy symptoms on hypoallergenic extensively hydrolyzed and hypoallergenic amino acid-based formulas, until she was switched to Alfamino®Infant
- The issues remained resolved, including during supplementary feeding, until 13 months of age when Alfamino® Infant had been discontinued for 4 weeks
References:
1. Koletzko S et al. Diagnostic approach and management of cow’s-milk protein allegy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55:221-229.
2. American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106:346-349.
Unless otherwise noted all trademarks are registered trademarks of Société des Produits Nestlé S.A., Vevey, Switzerland. ©2017 Nestlé Health Science. All rights reserved.
Funding for this case study was provided by Nestlé Health Science.